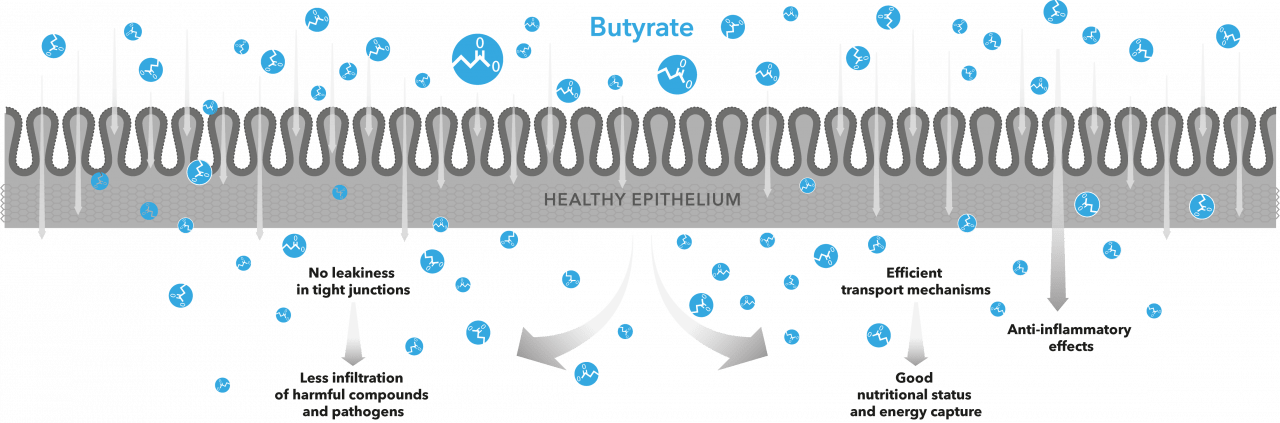
Butyrate, a short-chain fatty acid (SCFA) of 4-carbon atoms, is generally considered to be the preferred energy source of epithelial cells in the large intestine. Once produced by bacterial metabolism in the intestinal lumen, much of the butyrate is absorbed, to benefit the host animal’s energy status.
Origins of butyrate
The large intestine in most warm-blooded animals is comprised of caecum, colon and rectum. These three compartments are also the most bacteria-dense segments in the whole alimentary tract. In the intestinal lumen, digesta flows steadily towards the anus while being anaerobically fermented by a legion of bacteria, some of which are capable of butyrate production.
Butyrate and the intestinal cell types
Lining the intestine are the intestinal epithelial cells, which are an assortment of different cell types. These intestinal epithelial cells are the first to detect, transport and utilise the molecules present in the digesta. As these epithelial cells are of different types, they also respond differently to molecules they encounter. Enterocytes form the majority of epithelial cells in the intestine. In the large intestine, enterocytes have the important task of taking up ions and extracting water from the digesta. These cellular functions are energised by SCFAs in digesta. Another group of epithelial cells, the enteroendocrine cells (EECs), are present in small quantities throughout the alimentary tract. In the large intestine, three different types of EECs are present: L-cells, D-cells and EC-cells. The function of EECs is to sense bacterial metabolites and other molecules from the digesta. The EECs have vesicles that contain signalling molecules, which are released from the cell into extracellular space upon detection of a suitable metabolite, such as butyrate. The released signalling molecules are then detected by cells that have corresponding receptors on their cell membranes.
Butyrate and cellular signalling
The molecules utilised in cellular signalling in the large intestine are serotonin, somatostatin, peptide YY (PYY), glucagon-like peptide 1 and 2 (GLP-1 and GLP-2), oxyntomodulin and glicentin. These are molecules that can be detected in blood and have hormone-like action throughout the animal. Of the signalling molecules, PYY and GLP-2 have been shown to increase upon butyrate exposure in cell culture models and in human blood samples after meal consumption. PYY and GLP-2 stimulate proliferation of mucosal enterocytes and PYY also suppresses appetite. Both PYY and GLP-2 are secreted by the L-type of EECs, the number of which increases in the rectum, and thus they may be quick to sense and respond to changes in butyrate concentration in luminal digesta.
The EECs have also another way of communicating; they have been shown to form synapse-like contact sites with nerve terminals that reside in the intestinal mucosa. The release of signalling molecules from the EEC activates the neuron and the nerve impulses may then travel directly to other parts of the intestine via the enteric nervous system, and also to the central nervous system. Activity of the enteric neurons regulates intestinal motility and has effects on intestinal immunity by innervating gut-associated lymphoid tissue, while signals to the central nervous system affect appetite and even emotions. Thus, the signals from microbial metabolites activate a cascade of events which, through EECs signalling molecules and intestinal neurons, help in maintaining organismal homeostasis.
References to literature upon request.